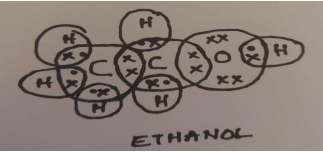
A carbon compound ‘A’ having melting point 156K and boiling point 351K, with molecular formula C2H6O is soluble in water in all proportions.
CBSE Sample Question Paper, Class 10 Science Term 2 Question 1 - (A carbon compound ‘A’ having melting point 156K and boiling point 351K, with molecular formula C2H6O is soluble in water in all proportions.)