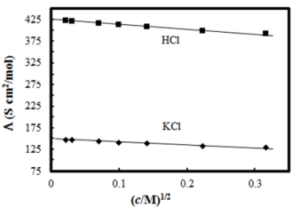
The molar conductivity of CH3COOH at infinite dilution is 390 Scm2 /mol. Using the graph and given information, the molar conductivity of CH3COOK will be:
Class 12th Chemistry, Question -The molar conductivity of CH3COOH at infinite dilution is 390 Scm2 /mol. Using the graph and given information, the molar conductivity of CH3COOK will be: