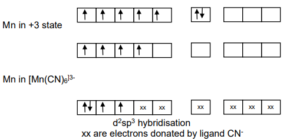
Using Valence bond theory, explain the following in relation to the paramagnetic complex [Mn(CN)6] 3-
Class 12th Chemistry, Question -Using Valence bond theory, explain the following in relation to the paramagnetic complex [Mn(CN)6] 3-
Class 12th Chemistry, Question -Using Valence bond theory, explain the following in relation to the paramagnetic complex [Mn(CN)6] 3-