Question :(i) It is observed that covalent compounds are bad conductors of electricity. Give reason.
(ii)Carbon can neither form C cation nor C* anion. Why?
(iii)fit Draw the electron dot structure of Ethanol.
(iv) Identify hetero atom(s) in the following compounds:
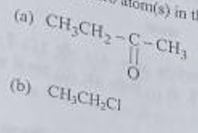
The correct answer is -(i) Covalent compounds are bad conductors of electricity because they do not have free electrons or ions that can move and carry an electric current. (ii) Carbon does not form C cation because it has a high ionization energy, which means it requires a lot of energy to remove an electron from it. Similarly, it does not form C* anion because it has a high electron affinity, which means it does not readily accept electrons to form an anion. (iii) The electron dot structure of Ethanol is as follows: H | H-C-O-H | H (iv) Hetero atoms in the following compounds are:
-
CH3NH2: Nitrogen
-
HCOOH: Oxygen


