Chapter 9 – Coordination Compounds Questions and Answers: NCERT Coordination Compounds for Class 12 Chemistry
Class 12 Chemistry chapter 9 - Coordination Compounds - Questions and Answers of NCERT Book Solutions
9.1. Write the formulas for the following coordination compounds:
(i)Tetraamminediaquacobalt(IlI) chloride
(ii)Potassium tetracyanidonickelate(II)
(iii)Tris(ethanp-1,2-diamine) chromium(III) chloride
(iv)Amminebromidochloridonitrito-N- platinatc(II)
(v)Dichloridobis(ethane-l ,2-diamine) platinum (IV) nitrate
(vi)Iron(III)hexacyanidoferrate(II)
Ans: (i) [CO(NH3)4(H2O)2]Cl3.
(ii)K2[Ni(CN)4]
(iii)[Cr(en)3]Cl3
(iv)[Pt (NH3) Br Cl (N02)]–
(v)[PtCl2(en)2](N03)2
(vi)Fe4[Fe(CN)6]3
9.2. Write IUPAC names of following co-ordination compounds :
(a) [CO(NH3)6]Cl3
(b) [CO(NH3)Cl]Cl2
(C) K3[Fe(CN)6]
(d) [K3[Fe(C2O4)3]
(e) K2[PdCl4]
(f) [Pt(NH3)2ClNH2CH3]Cl.
Ans:
(a) hexaamminecobalt (III) chloride
(b) pentaamminechloridocobalt (III) chloride
(c) potassium hexacyanoferrate (III)
(d) potassium trioxalatoferrate (III)
(e) potassium tetrachloridoplatinum (II)
(f) diamminechlorido (methylamine) platinum(II) chloride.
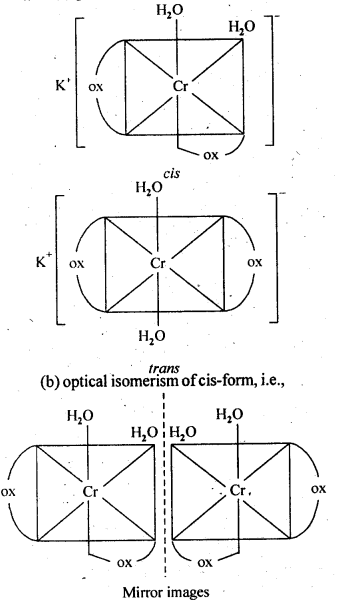
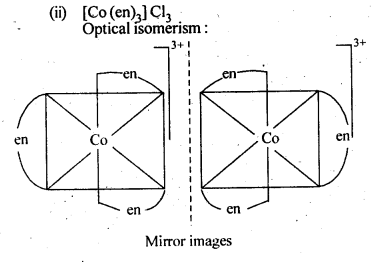
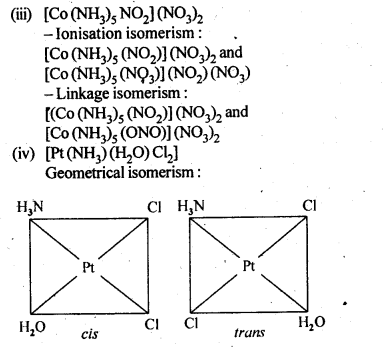
9.3. Indicate the types of isomerism exhibited by the . . following complexes and draw the structures for these isomers:
(i)K[Cr(H2O)2(C2O4)2]
(ii)[CO(en)3]Cl3
(iii)[CO(NH3)5(NO2)(NO3)2], .
(iv)[Pt(NH3)(H2O)Cl2]
Ans: (i)(a) geometrical isomerism (cis and tram)
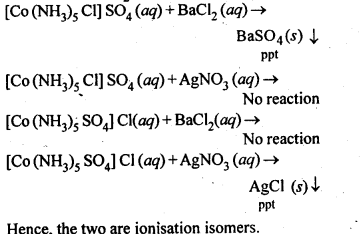
9.4. Give evidence that [Co(NH3)5Cl]S04 and [Co(NH3)5S04]Cl are ionisation isomers.
Ans: When dissolved in water, they give different ions in solution which can be tested by adding AgN03 solution and BaCl2 solution, i.e.,
9.5. Explain on the basis of valence bond theory that [Ni(CN)4]2- ion with square planar structure is diamagnetic and [NiCl4]2- ion with tetrahedral geometry is paramagnetic.
Ans: Outer electronic configuration of nickel (Z = 28) in ground state is 3d84s2. Nickel in this complex is in + 2 oxidation state. It achieves + 2 oxidation state by the loss of the two 4s-electrons. The resulting Ni2+ ion has outer electronic configuration of 3d8. Since CN– ion is a strong field, under its attacking influence, two unpaired electrons in the 3d orbitals pair up.
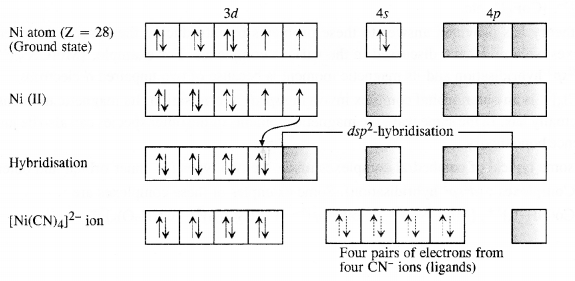
Outer electronic configuration of nickel (Z = 28) in ground state is 3d84s2 Nickel in this complex is in + 2 oxidation state. Nickel achieves + 2 oxidation state by the loss of two 4s-electrons. The resulting Ni2+ ion has outer electronic configuration of 3d8. Since CP ion is a weak field ligand, it is not in a position to cause electron pairing.
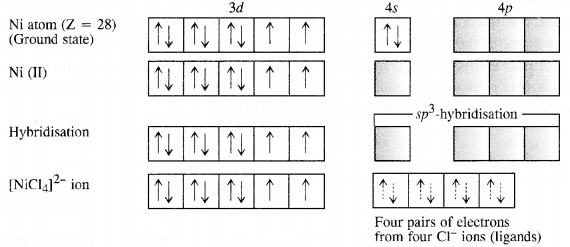
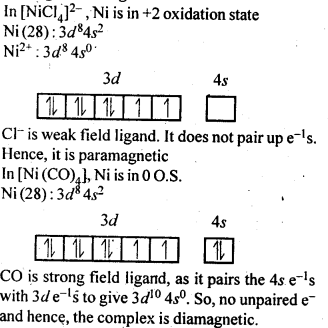
9.6. [NiCl4]2- is paramagnetic while [Ni(CO)4] is diamagnetic though both are tetrahedraL Why?
Ans:
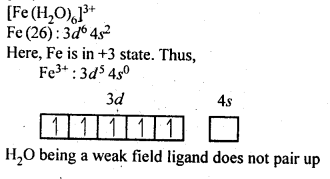
9.7. [Fe(H2O)6]3+is strongly paramagnetic whereas [Fe(CN)6]3-is weakly paramagnetic. Explain.
Ans:
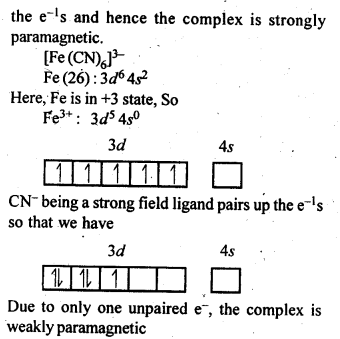
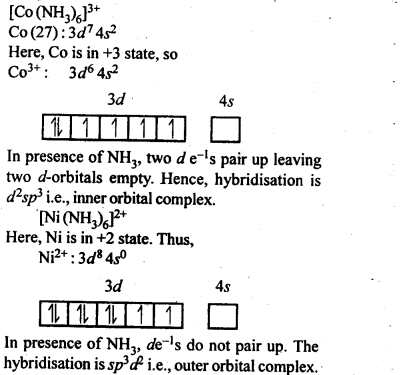
9.8. Explain[CO(NH3)6]2+ is an inner orbital complex.whereas [Ni(NH3)6]2+ is an outer orbital complex.
Ans:
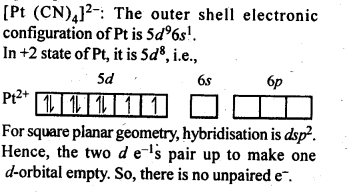
9.9. Predict the number of unpaired electrons in the square planar [Pt(CN)4]2-ion.
Ans:
9.10. The hexaaquamanganese (II) ion contains five unpaired electrons while the hexacyano ion contains only one unpaired electron. Explain using crystal field theory.
Ans: Mn(II) ion has 3d5 configuration. In the presence of H2O molecules acting as weak field ligands, the distribution of these five electrons is t32ge2 i. e., all the electrons remain unpaired to form a high spin complex. However, in the presence of CN– acting as strong field ligands, the distribution of these electrons is t52ge0g i.e., two t2g orbitals contain paired electrons while the third t2g orbital contains one unpaired electron. The complex formed is a low spin complex.
9.11. Calculate the overall complex dissociation equilibrium constant for the Cu(NH3)42+ ion, given that β4 for this complex is 2.1 x 1013.
Ans: Overall stability constant (β4) = 2.1 x 1013.
Thus, the overall dissociation constant is
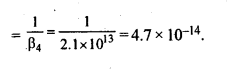
Last Updated on: January 14, 2026
