Chapter 8 – The d and f Block Elements Questions and Answers: NCERT The d and f Block Elements for Class 12 Chemistry
Class 12 Chemistry chapter 8 - The d and f Block Elements - Questions and Answers of NCERT Book Solutions.
8.1. Silver atom has completely filled d orbitals (4d10) in its ground state. How can you say that it is a transition element?
Ans: The outer electronic configuration of Ag (Z=47) is 4d105s1. It shows+1 and + 2 O.S. (in AgO and AgF2). And in + 2 O.S., the electronic configuration is d9 i.e, d-subshell is incompletely filled. Hence, it is a transition element.
8.2. In the series Sc(Z = 21) to (Z = 30), the enthalpy of atomisation of zinc is the lowest i.e., 126 kJ mol-1. Why?
Ans: The enthalpy of atomisation is directly linked with the stability of the crystal lattice and also the strength of the metallic bond. In case of zinc (3d104s2 configuration), no electrons from the 3d-orbitals are involved in the formation of metallic bonds since all the orbitals are filled. However, in all other elements belonging to 3d series one or more d-electrons are involved in the metallic bonds. This means that the metallic bonds are quite weak in zinc and it has therefore, lowest enthalpy of atomisation in the 3d series.
8.3. Which of the 3d series of the transition metals exhibits the largest number of oxidation states and why?
Ans: Manganese (Z = 25) shows maximum number of O.S. This is because its outer EC is 3d54s2. As 3d and 4s are close in energy, it has maximum number of e-1 s to loose or share. Hence, it shows O.S. from +2 to +7 which is the maximum number.
8.4.
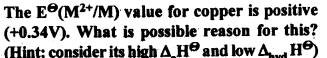
Ans.
8.5. How would you account for the irregular variation of ionisation enthalpies (first and second) in the first series of the transition elements?
Ans: There is a irregularity in the IE’s of 3d-series due to alternation of energies of 4s and 3d orbitals when an e-1 is removed. Thus, there is a reorganisation energy accompanying ionization. This results into release of exchange energy which increases as the number of e-1 s increases in the dn configuration. Cr has low 1st IE because loss of 1 e- gives stable EC (3d6). Zn has very high IE because e~ has to be removed from 4s orbital of the stable configuration (3d10 4s2) After the loss of one e–, removal of 2nd e–, becomes difficult. Hence, 2nd IE’s are higher and in general, increase from left to right. However, Cr and Cu show much higher values because 2nd e– has to be removed from stable configuration of Cr+ (3d5) and Cu+ (3d10)
8.6. Why is the highest oxidation state of a metal exhibited by its fluoride and oxide only?
Ans: Both fluorine and oxygen have very high electronegativity values. They can oxidise the metals to the highest oxidation state. As a result, the highest oxidation states are shown by the fluorides and oxides of the metals; transition metals in particular.
8.7.Which is a stronger reducing agent Cr2+ or Fe2+ and why?
Ans: Cr2+ is a stronger reducing agent than Fe2+. This is because E°(Cr3+/Cr2+) is negative (- 0.41V) whereas E°(Fe3+/Fe2+) is positive (+ 0.77 V). Thus, Cr2+ is easily oxidised to Fe3+ but Fe2+ cannot be easily oxidised to Fe3+.
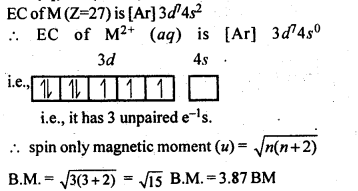
8.8.Calculate the ‘spin only’ magnetic moment of M2+(aq) ion (Z = 27).
Ans:
8.9.Explain why Cu+ ion is not stable in aqueous solutions?
Ans: Cu+ (aq) is not stable, while Cu2+ (aq) is stable. This is becuase ΔhydH of Cu2+(aq) is much higher than that of Cu+(aq) and hence it compensates for the 2nd IE of Cu. Thus, many Cu(I) compounds are unstable in aqueous solution and undergo disproportionation as follows :
2 Cu+ —–> Cu2+ + Cu
8.10. Actinoid contraction is greater from element to element than lanthanoid contraction. Why?
Ans: The decrease or contraction in atomic radii, as well as ionic radii in actinoid elements (actinoid contraction), is more as compared to lanthanoid contraction because 5/ electrons have more poor shielding effect as compared to 4f electrons. Therefore, the effect of increased nuclear charge leading to contraction in size is more in case of actinoid elements.
Last Updated on: January 14, 2026
