Chapter 6 – General Principles and Processes of Isolation of Elements Questions and Answers: NCERT General Principles and Processes of Isolation of Elements for Class 12 Chemistry
Class 12 Chemistry chapter 6 - General Principles and Processes of Isolation of Elements - Questions and Answers of NCERT Book Solutions.
6.1. Which of the ores mentioned can be concentrated by magnetic separation method?
Ans: Ores Which are magnetic in nature can be separated from non-magnetic gangue particles by magnetic separation method. For ex: ores of iron such as haemetite (Fe2O3), magnetite (Fe3O4), siderite (FeCO3) and iron pyrites (FeS2) being magnetic can be separated from non-magnetic silica and other impurities by magnetic separation method.
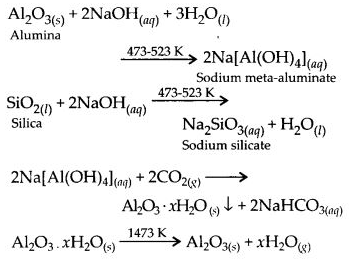
6.2. What is the significance of leaching in the extraction of aluminium?
Ans: Leaching or chemical separation is quite effective to purify bauxite an ore of aluminium associated with the impurities of iron oxide. The ore is leached with concentrated solution of NaOH to form a soluble complex leaving behind the impurities.
6.3.
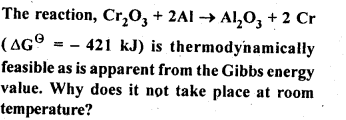
Ans: This is explained on the basis of Keq, the equilibrium constant. In the given redox reaction, all reactants and products are solids at room temperature, so, there is no equilibrium between the reactants and products and hence the reactions does not occur at RT. At high temperature, Cr melts and values of TAS increases. As a result, the value of
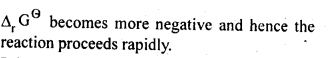
6.4. Is it true that under certain conditions, Mg can reduce Al203 and Al can reduce MgO? What are those conditions?
Ans:
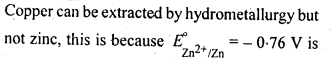
Last Updated on: January 14, 2026
